Overview of the CRISPR IP Landscape
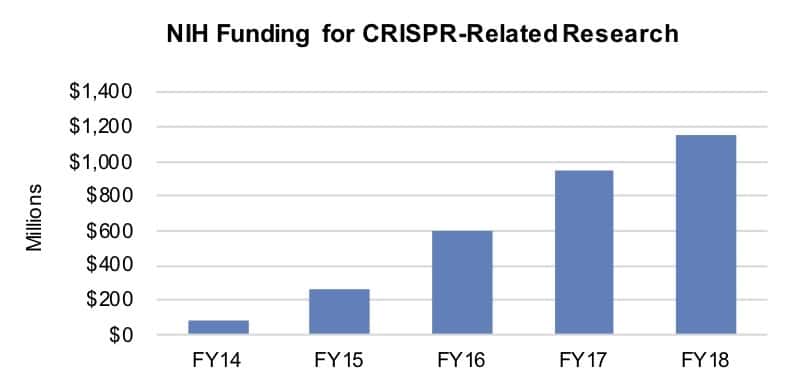
CRISPR gene-editing technology has rapidly emerged as one of the most transformative innovations in genomics, blending cutting-edge science with significant commercial potential[1]. The technology's ability to precisely edit genes using the CRISPR-associated protein 9 (Cas9) has not only accelerated research across human health, agriculture, and biotechnology, but has also expanded licensing activities and competitive patent battles worldwide[1]. With over 1,700 patents filed and hundreds of institutions and companies involved, the CRISPR arena is mired in overlapping claims and evolving legal interpretations which now play a critical role in the commercialization and further development of this technology[5].
Major Court Cases and Regional Differences
In the United States, a series of appellate decisions have influenced the determination of patent rights. A landmark decision by a US appeals court recently revived the bid of the University of California and the University of Vienna—represented by pioneers Doudna and Charpentier—to claim exclusive rights to the CRISPR–Cas9 technology, overturning prior decisions that favored the Broad Institute[7]. This appeal has led to the case being sent back to the Patent Trial and Appeal Board for re-evaluation of competing claims, thereby reopening the discussion on priority and inventorship in the US market[7]. Meanwhile, in Europe, the patent landscape exhibits distinct outcomes. The European Patent Office (EPO) has witnessed significant developments, including the voluntary withdrawal of certain foundational CRISPR patents by the CVC group after an unfavorable opinion and the subsequent notification to grant broader divisional applications for CRISPR-related claims[4]. Additionally, the Board of Appeals of the EPO reversed earlier decisions that limited Broad Institute patents by affirming their priority claims based on earlier US provisional applications, and these cases have been remanded for further prosecution[8]. In Asia, regional rulings have further diversified the landscape with Japan's IP High Court upholding key CRISPR patents for the CVC group and Chinese authorities endorsing fundamental CRISPR patents, thereby reflecting pronounced regional differences in patent enforcement and priority determination[4].
Commercialization, Investment, and Collaborative Research Implications
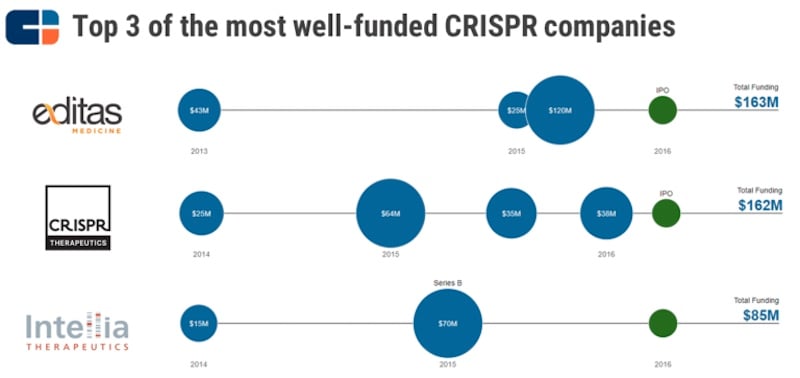
The ongoing patent disputes and varied regional rulings have significant implications for commercialization and investment. Licensing negotiations are becoming increasingly complex as companies must now consider whether the final resolution of these disputes will grant licenses from both the prevailing and losing groups[3]. Licensees who have previously arranged terms with the losing group might face challenges in negotiating equivalent scope, exclusivity, or financial conditions with the prevailing patent holders once the dispute is resolved, potentially leading to increased royalty costs and stricter licensing conditions[3]. Investment has been robust despite these uncertainties, as leading companies in the CRISPR space—such as CRISPR Therapeutics, Editas Medicine, and Intellia Therapeutics—have attracted significant venture capital and public funding, underscoring the belief that the technology's potential outweighs the risks associated with the patent battles[5].
Collaborative research and strategic partnerships are also being reshaped by the patent landscape. For instance, numerous high-value agreements have been formed between biotech firms and pharmaceutical companies, with deals potentially valued in the billions, reflecting the high commercial stakes and the need for access to diverse patent portfolios to ensure global coverage[1]. Furthermore, the potential formation of a patent pool has been discussed as a means to streamline licensing, reduce the risk of royalty stacking, and facilitate more inclusive access to essential CRISPR technologies, although challenges remain in achieving consensus among all the major patent holders[4].
These dynamics have a direct influence on investment decisions and research collaborations. Investors require a clear intellectual property strategy to mitigate risks, while research institutions and companies must tailor their licensing strategies to navigate the evolving legal environment. As long as the disputes persist, stakeholders are compelled to closely monitor litigation outcomes, adjust contractual terms, and engage in active dialogue with patent owners to secure the necessary rights for innovation and market entry[3].
Get more accurate answers with Super Pandi, upload files, personalized discovery feed, save searches and contribute to the PandiPedia.
Let's look at alternatives:
- Modify the query.
- Start a new thread.
- Remove sources (if manually added).