The Dawn of Personalized Nutrition
Personalized nutrition, also known as nutrigenomics, is an approach that tailors dietary advice and interventions based on an individual's unique genetic profile, health status, lifestyle, and preferences[1][17]. This marks a significant shift from traditional, generalized dietary guidelines to a more individualized strategy[3][10]. The field studies the relationship between the human genome, nutrition, and health, with the goal of preventing chronic diseases and improving well-being and longevity[1][10][17]. As our understanding of genetics and metabolism advances, tailored nutrition is poised to significantly improve public health and usher in a new era of personalized medicine[1][17].
The Scientific Foundation: Linking Genes to Diet
The scientific basis for personalized nutrition lies in nutrigenomics, which examines how genetic variations influence metabolism and nutrient absorption[3]. Individual genetic differences, such as single nucleotide polymorphisms (SNPs), can affect how the body processes nutrients and impact susceptibility to conditions like obesity, diabetes, and cardiovascular disease[3][5]. For example, polymorphisms in the MTHFR gene affect folate metabolism, variations in APOE modify lipid metabolism, and certain SNPs in the FTO gene are associated with obesity[3][5]. This gene-nutrient interaction is a two-way street; not only do genetic variants influence the body's response to nutrients (nutrigenetics), but nutrients themselves can also modulate gene expression (nutrigenomics)[5]. However, the science is complex. Most diet-related diseases are not caused by a single gene but result from a combination of multiple genetic and environmental factors[5]. For instance, while the FTO gene is strongly linked to obesity, its most studied variant explains less than 1% of the variance in BMI, highlighting the limited predictive power of single-gene analyses for complex traits[5].
The Role of AI and Machine Learning in Crafting Dietary Plans
Artificial Intelligence (AI) and Machine Learning (ML) are revolutionizing nutrigenomics by enabling the analysis of vast and complex datasets[1][8][17]. AI-driven systems can integrate diverse data sources, including genomics, microbiome profiles, metabolic markers, medical history, and real-time data from wearable devices, to generate personalized dietary recommendations with unprecedented precision[3][8][12]. Supervised ML models like Support Vector Machines (SVM) and Random Forest (RF), along with unsupervised methods like Principal Component Analysis (PCA), are used to identify patterns, classify individuals, and predict responses to dietary interventions[6]. For example, ML was used to build a predictive model that identified two metabolites capable of predicting significant weight loss in subjects on a specific diet[1][17]. AI also powers applications that provide real-time feedback and dynamic adjustments to diet plans based on data from wearables like continuous glucose monitors, ensuring that recommendations evolve with an individual's changing health status[3][12].
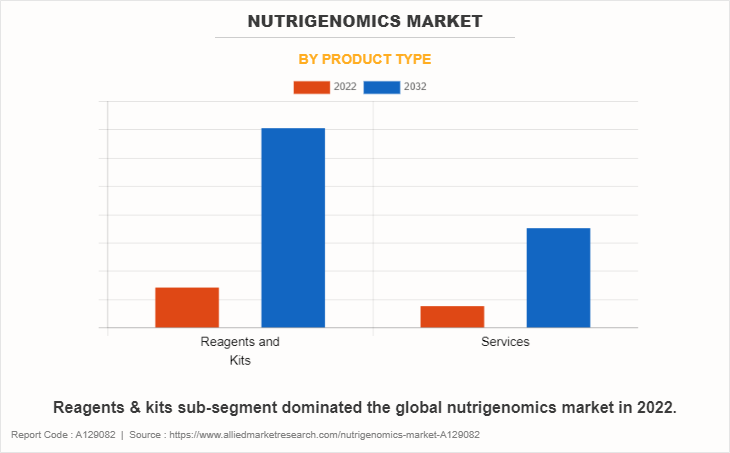
Business Models: Direct-to-Consumer (DTC) Services
Driven by falling DNA sequencing costs, a growing number of companies offer nutrigenomics testing services directly to consumers (DTC)[5][11][18]. The typical DTC model involves a consumer ordering a kit online, providing a saliva sample at home, and receiving a report with genetic predispositions and dietary advice, all without the supervision of a clinician[5][10]. These services often focus on weight management, nutrient metabolism, food intolerances (like lactose and caffeine), and risk assessment for diet-related disorders[5][10]. Research has identified nine dominant business model
Evaluating Scientific Validity and Key Challenges

Despite the promise of personalized nutrition, the scientific validity of many commercial DTC tests is a significant concern[7][10]. One of the main challenges is the inadequate interpretation of complex genetic data, particularly when delivered without guidance from a qualified healthcare provider[10]. Studies have found conflicting findings and a lack of a consistent, sound evidence base for the gene-diet associations used in some commercial tests[7]. A 2020 analysis of 45 DTC companies revealed that 29 of them (about two-thirds) did not provide any information about the specific genes or genetic variants they analyzed, making it impossible to evaluate their scientific reliability[5]. Furthermore, many companies base predictions for highly complex traits on just a few genetic variants, which have very limited predictive ability[5]. The development of AI solutions also faces hurdles, including the
Navigating the Regulatory and Ethical Landscape
The personalized nutrition industry operates in a complex and evolving regulatory environment[4]. Regulations are often not written to cover personalized nutrition programs as a whole, but rather their individual components, such as devices, nutrition products, and health claims, which can be confusing[4]. The lack of comprehensive and harmonized global regulations poses a significant challenge for companies[14]. Key ethical concerns center on the collection and storage of sensitive genetic information, raising issues of data privacy, security, informed consent, and the potential for genetic discrimination[3][10][14]. To address these issues, experts have called for regulatory guidance focusing on the safety and accuracy of tests and responsible communication of benefits[4]. Suggestions include establishing a network of regulatory experts to support companies and creating a certification process to help consumers identify high-quality, trustworthy services[4]. Adherence to new frameworks like the EU's AI Act and the US's AI Bill of Rights will also be vital to ensure that AI systems are safe, transparent, and non-discriminatory[15].
Get more accurate answers with Super Pandi, upload files, personalized discovery feed, save searches and contribute to the PandiPedia.
Let's look at alternatives:
- Modify the query.
- Start a new thread.
- Remove sources (if manually added).