Overview
AI‐enabled wearable devices are increasingly becoming part of the medical device landscape, offering advanced functionalities ranging from continuous health monitoring to early diagnostic assistance. In the United States, manufacturers face critical regulatory decisions when seeking FDA clearance, as they must determine whether their device can leverage an existing predicate or if it is sufficiently novel to require a different regulatory pathway. The FDA employs a risk‐based system that not only assesses the premarket evidence required for approval, but also emphasizes post‐market surveillance and lifecycle management of AI software functions[3][14].
Regulatory Pathways: 510(k) Versus De Novo

For AI‐enabled wearables, two primary FDA clearance pathways are available: the 510(k) and the De Novo routes. The 510(k) process is based on demonstrating substantial equivalence to a legally marketed predicate device. In this process, the new device is compared against an existing device that has already been cleared, meaning that if the similarities in design, intended use, and technological characteristics align, the pathway tends to be faster and less resource intensive[3]. Conversely, if the wearable incorporates novel machine‐learning algorithms or unique interactions that lack a clear predicate, manufacturers may need to pursue the De Novo pathway. The De Novo process essentially establishes a new device classification when general and special controls are sufficient to ensure safety and effectiveness but no predicate exists[12]
The choice between these pathways hinges on the degree of innovation and the presence of a similar device on the market. Some manufacturers initially attempt to use a predicate to benefit from the predictability of the 510(k) process; however, when the predicate does not clearly match the new wearable's capabilities, the De Novo pathway becomes the logical choice despite its typically longer review cycle[12].
Clinical Evidence Requirements
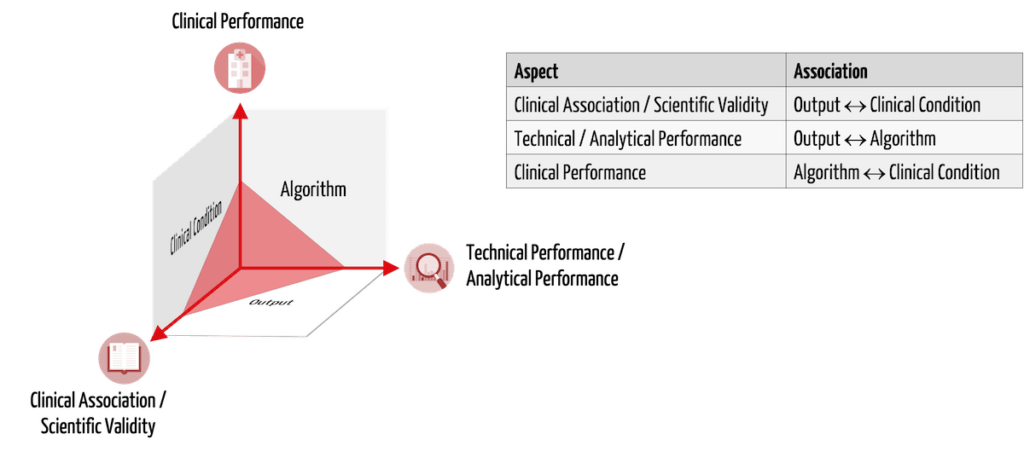
Regardless of the pathway, clinical evidence remains a cornerstone of the FDA's evaluation for medical devices, including AI‐enabled wearables. For devices cleared via the 510(k) process, clinical studies may be less burdensome if the predicate device carries already established performance data; however, it is critical to demonstrate that the new device maintains similar sensitivity, specificity, and overall performance in its clinical use setting[3]. In contrast, the De Novo route often requires the submission of robust clinical evidence, sometimes involving prospective clinical studies, to affirm that the benefits outweigh the potential risks, given the novelty of the technology[12].
Moreover, for wearables that incorporate AI algorithms, challenges such as demonstrating the equivalence of software functionalities or mitigating biases inherent in machine learning models become central issues. In many instances, manufacturers may leverage retrospective analyses or previously published data to support performance claims, although this approach is more feasible when the data sets are robust, independent, and representative of the intended patient population[5].
Post‐Market Surveillance and Lifecycle Management
Once approved, AI‐enabled wearables are subject to comprehensive post‐market surveillance to ensure that their performance, safety, and effectiveness are maintained under real‐world conditions. Post‐market surveillance activities include systematic data collection from adverse event reports, complaint handling, and regular performance evaluations in the field[8]. The FDA expects manufacturers to implement a robust plan for post‐market clinical follow‐up (PMCF), which feeds into the ongoing clinical evaluation process to detect any issues that may arise once the device is in regular use[13].
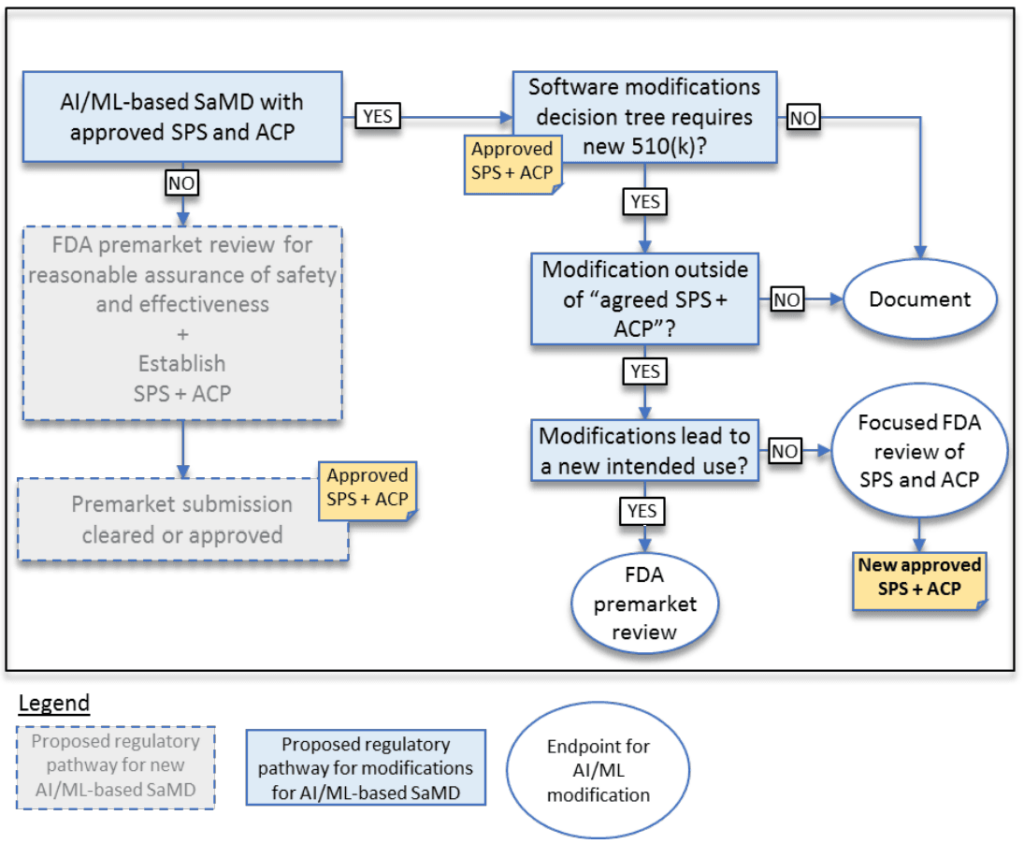
It is also recommended that manufacturers adopt a proactive approach by including a Predetermined Change Control Plan (PCCP) as part of their submission. This approach allows for certain modifications to the AI software – such as improved performance or updates to the model – to be made without the need for a complete resubmission, provided these changes remain within the predefined parameters. The PCCP requires a detailed framework for how updates will be managed, including data management, retraining protocols, risk mitigation strategies, and user communication plans[15].
Additionally, the FDA's total product lifecycle (TPLC) approach mandates that manufacturers continuously monitor and report on real‐world performance, ensuring that any deviations from expected behavior are promptly addressed through robust quality management systems and cybersecurity measures[18].
Key Considerations for AI-Enabled Wearables
Manufacturers developing AI‐enabled wearable devices must address several interrelated challenges. They need to ensure that the device's machine learning algorithms are trained on diverse and representative data to mitigate potential biases that could affect performance. This is particularly important when intending to compare the wearable's performance against a predicate device in a 510(k) submission or when establishing entirely new indicators of performance for a De Novo submission[19].
Furthermore, regulatory submissions must clearly articulate the intended use, user interface, and integration of the device into clinical workflows. Clear labeling is also critical to inform healthcare providers and patients about the functioning of the AI-enabled system, including any automated functions, operating conditions, and limitations inherent to the technology[14].
Continuous risk management remains a vital aspect throughout the device's lifecycle. Manufacturers must not only validate the initial performance based on premarket studies but also set up effective monitoring systems to capture any safety signals post-authorization. This ongoing commitment to safety and performance is essential to address any unforeseen issues and to comply with the stringent requirements outlined by the FDA[13].
Conclusion
In summary, navigating FDA clearance for AI‐enabled wearables requires a strategic evaluation of the available regulatory pathways. Manufacturers must decide between using the 510(k) process, which relies on predicate comparability, and the De Novo pathway, which is tailored for innovative devices lacking similar predecessors. Both pathways demand robust clinical evidence to substantiate claims of safety and effectiveness, with the level of evidence typically higher for devices cleared via De Novo. Post‐market surveillance and a proactive lifecycle management approach – including the use of tools like the Predetermined Change Control Plan – are integral to maintaining device performance and safety in real‐world settings. By addressing these key regulatory considerations, manufacturers can ensure that their AI‐enabled wearable devices not only gain FDA clearance but also continue to deliver safe and effective performance over time[3][15][18].
Get more accurate answers with Super Pandi, upload files, personalized discovery feed, save searches and contribute to the PandiPedia.
Let's look at alternatives:
- Modify the query.
- Start a new thread.
- Remove sources (if manually added).