Antibiotics are crucial tools in the treatment of bacterial infections, employed to eradicate or inhibit the growth and reproduction of harmful microorganisms. They function through various mechanisms that specifically target bacterial physiology, making them less harmful to human cells. Understanding how these agents work is essential, especially in the face of rising antibiotic resistance.
Targeting Unique Bacterial Structures

Many antibiotics exploit the unique structures found in bacterial cells that are absent in human cells. For instance, most bacteria possess a cell wall made of peptidoglycan, which is vital for maintaining their shape and integrity. Antibiotics such as penicillin interfere with the synthesis of this cell wall. Penicillin specifically blocks the transpeptidation step in peptidoglycan assembly, leading to a fragile cell wall that cannot withstand osmotic pressure, ultimately causing the bacterial cell to burst and die[6][7].
Beta-lactams, a major class of antibiotics that includes penicillin, mimic the molecular structure of the D-alanyl-D-alanine portion of peptidoglycan precursors, allowing them to bind to penicillin-binding proteins (PBPs) essential for cell wall synthesis. This binding inhibits the enzyme's function and disrupts peptidoglycan layer formation, leading to bacterial lysis[2][5].
Inhibition of Protein Synthesis

Antibiotics can also inhibit bacterial growth by targeting protein synthesis. Ribosomes, the cellular machinery for protein production, differ between human and bacterial cells, allowing antibiotics to selectively disrupt bacterial protein synthesis. For example, aminoglycosides bind to the 30S ribosomal subunit, causing misreading of mRNA and premature termination of protein synthesis, which leads to cell death[2][3][7]. Tetracyclines operate through a similar mechanism, blocking the access of aminoacyl-tRNA to the ribosome, effectively halting translation[2][3].
Notably, the bactericidal effect of certain ribosome-targeting antibiotics is not solely due to halting protein synthesis but may also involve triggering oxidative damage pathways. Aminoglycosides, for instance, can induce toxic mistranslated proteins that disrupt membrane integrity, contributing to cell death[1][4][5].
Disruption of Nucleic Acid Synthesis
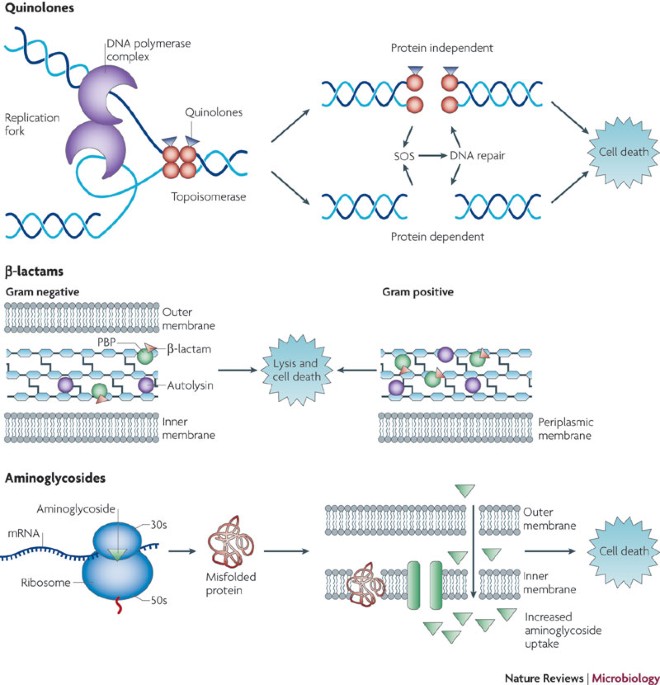
Other antibiotics, such as rifampicin and quinolones, target nucleic acids. Rifampicin inhibits DNA-dependent RNA polymerase, blocking the initiation of transcription and subsequently leading to protein synthesis cessation. This impact on transcription can produce rapid bactericidal effects, particularly in slowly growing bacteria[3][5][6].
Quinolones, including fluoroquinolones, disrupt DNA replication by inhibiting topoisomerases, enzymes critical for maintaining DNA structure. They interfere with the action of DNA gyrase and topoisomerase IV, leading to the formation of stable drug-enzyme-DNA complexes that prevent DNA unwinding, essential for replication and transcription[1][3][5]. This ultimately results in bacterial cell death via mechanisms associated with DNA damage and the activation of stress response pathways, particularly the SOS response, which can lead to further complications for the bacterial cell[1][3][4].
Oxidative Stress and Cell Death
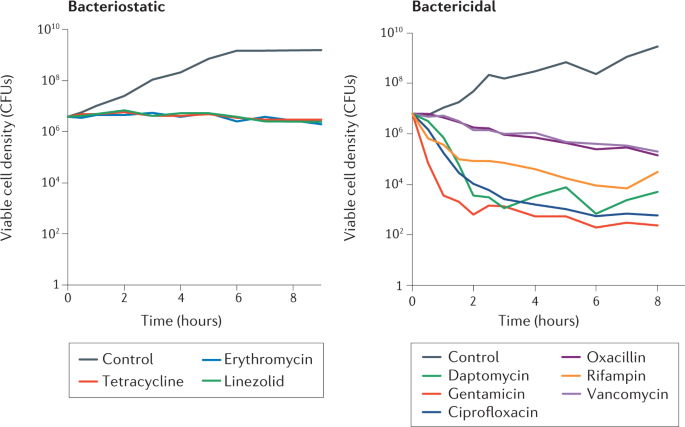
Increasing evidence points to a common mechanism by which various classes of bactericidal antibiotics induce cell death, primarily through the generation of reactive oxygen species (ROS). When bacteria are exposed to lethal concentrations of antibiotics, metabolic alterations can result in oxidative stress, producing harmful superoxide and hydroxyl radicals. This oxidative damage can impair various cellular components, including DNA and proteins, contributing to cell lysis and death[1][4].
For instance, research indicates that different antibiotics can stimulate ROS production via drug-induced changes in central metabolism, leading to the generation of cytotoxic hydroxyl radicals[1][4][5]. This pathway underscores the complexity of antibiotic action, as it highlights the interplay between direct antibacterial effects and cellular stress responses.
The Challenge of Antibiotic Resistance
Despite the advances in understanding antibiotic mechanisms, bacterial resistance is an escalating challenge. Bacteria can adapt through various mechanisms, such as modifying drug targets, producing enzymes that deactivate antibiotics, or enhancing efflux pumps to expel the drugs more effectively. For example, mutations in PBPs can confer resistance to beta-lactam antibiotics, making treatment more challenging[2][6][8].
Antibiotic stewardship, including proper usage based on sensitivity testing, is vital in managing the development of resistance. Understanding the mechanisms by which antibiotics work can aid in the development of new agents and strategies to combat antibiotic-resistant infections. Continued research into the molecular interactions and metabolic pathways affected by antibiotics remains critical for advancing treatment methodologies and ensuring patient safety[3][4][7][8].
Conclusion
Antibiotics function through diverse and intricate mechanisms that exploit the distinct characteristics of bacterial cells. By disrupting cell wall synthesis, inhibiting protein and nucleic acid production, and inducing oxidative stress, these compounds effectively combat bacterial infections. However, the rise of antibiotic resistance highlights the need for ongoing research and prudent use of these vital medications. Understanding antibiotic mechanisms is essential not only for current therapeutic strategies but also for developing innovative approaches to future bacterial infections.
Get more accurate answers with Super Pandi, upload files, personalized discovery feed, save searches and contribute to the PandiPedia.
Let's look at alternatives:
- Modify the query.
- Start a new thread.
- Remove sources (if manually added).