The Unique Properties of Water Essential for Life
Water is often referred to as the 'mother liquid' of life, and its biological significance stems from a range of unique properties that make it indispensable for living organisms. These properties directly contribute to the basic functions necessary for survival and the complexity of life forms.
Polarity and Solvent Abilities
One of water's most critical characteristics is its polarity, which leads to its exceptional ability as a solvent. The water molecule consists of two hydrogen atoms bonded to one oxygen atom, and this configuration creates a polarity where the hydrogen side is positively charged and the oxygen side is negatively charged. This polarity allows water to dissolve a wide variety of substances, enabling it to carry essential ions and nutrients throughout biological systems. As stated, 'water is this wonderful universal solvent,' meaning almost every substance can dissolve in water, which makes it an effective medium for cellular transport and biochemical reactions[2].
High Heat Capacity and Regulation of Temperature
Another vital property of water is its high heat capacity. Water can absorb significant amounts of heat without a corresponding large increase in temperature. This quality allows organisms to maintain stable internal temperatures despite external temperature fluctuations. The ability of water to stay liquid over a broad range of temperatures significantly contributes to the stability of ecosystems and supports diverse habitats. As noted, 'water prevents the effects of temperature fluctuations in the surroundings'[1].
Cohesion and Adhesion
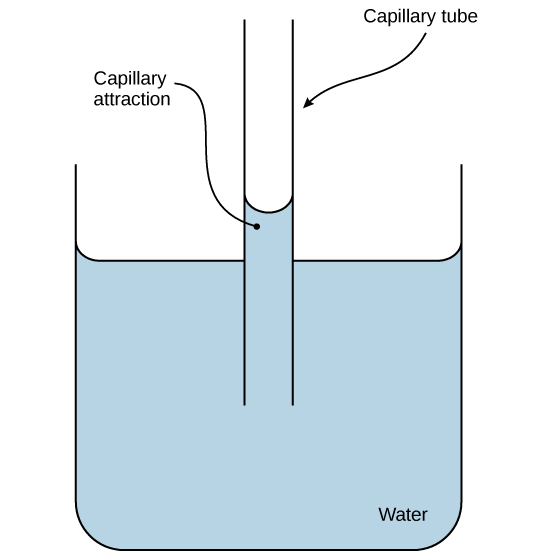
Water's unique hydrogen bonding leads to high cohesion, the attractive force between like molecules. This property is vital for creating surface tension, allowing certain organisms, like water striders, to walk on water without breaking the surface. Cohesion is crucial for the movement of water through plant xylem, helping transport water from roots to leaves. Water also demonstrates adhesion, the attraction between water molecules and different substances, facilitating processes like capillary action, which helps draw water upwards in plants[3].
Density and Ice Formation
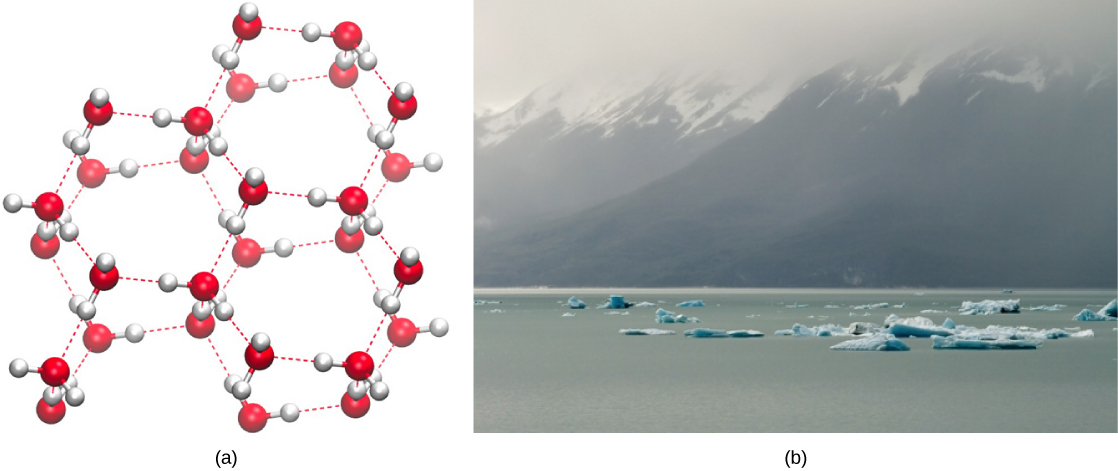
Water is unique in that it is less dense in its solid state than in its liquid state. This means that ice floats on liquid water, forming an insulating layer on bodies of water in winter, which protects aquatic life from extreme cold. The phenomenon that 'if water shrinks during freezing, the ice will sink... and will destroy the aquatic life in Polar Regions' highlights the importance of water's density for maintaining ecosystems[1]. The crystalline structure of ice, maintained by hydrogen bonds, creates an effective insulator, allowing life to thrive beneath the surface even in freezing temperatures.
Role in Biological Reactions
Water is not merely a passive medium but actively participates in various biological reactions. It acts as a reactant in processes such as hydrolysis, where it helps break down complex molecules into simpler ones necessary for metabolism. Additionally, during photosynthesis, oxygen is released by the hydrolysis of water[1]. The presence of water also assists in creating buffers which help maintain an essential pH level for biochemical processes, contributing to the overall homeostasis of living organisms[3].
Support for Life Systems and Organism Functionality
In living systems, approximately 70-90% of a cell's composition is water, emphasizing its crucial role in supporting cellular structure and function[1]. Water is involved in regulating osmotic pressure, sustaining cell turgidity, and providing a medium for nutrient transport, as it enables the diffusion of molecules within cells[1]. Furthermore, the lymphatic and excretory systems in animals utilize water to facilitate transport and waste removal, showing how integral water is to bodily functions.
Conclusion
In summary, the unique properties of water—its polarity, high heat capacity, moisture retention abilities, cohesive and adhesive qualities, density anomaly, and active participation in biological reactions—collectively make it an essential component of life on Earth. The interplay of these properties not only sustains individual organisms but also supports entire ecosystems, exemplifying why water is often hailed as the foundation of life. Understanding water's critical roles enhances our appreciation for this vital resource and its importance in the quest for life beyond our planet[2][3].
Get more accurate answers with Super Pandi, upload files, personalized discovery feed, save searches and contribute to the PandiPedia.
Let's look at alternatives:
- Modify the query.
- Start a new thread.
- Remove sources (if manually added).